
Energy can change from one form to another, but it can be neither created nor destroyed.

The reason is that the entropies listed are absolute, rather than relative to some arbitrary standard like enthalpy. Note that there are values listed for elements, unlike DH fº values for elements. The Thermodynamics Table lists the entropies of some substances at 25 ✬. Continue this process until you reach the temperature for which you want to know the entropy of a substance (25 ✬ is a common temperature for reporting the entropy of a substance). Then you can use equation (1) to calculate the entropy changes. Even though equation (1) only works when the temperature is constant, it is approximately correct when the temperature change is small. Now start introducing small amounts of heat and measuring the temperature change. Since there is no disorder in this state, the entropy can be defined as zero. Imagine cooling the substance to absolute zero and forming a perfect crystal (no holes, all the atoms in their exact place in the crystal lattice). The absolute entropy of any substance can be calculated using equation (1) in the following way.

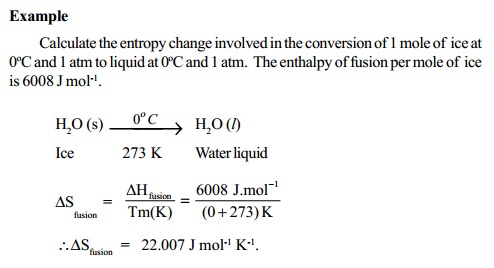
At absolute 0 (0 K), all atomic motion ceases and the disorder in a substance is zero. On this scale, zero is the theoretically lowest possible temperature that any substance can reach. The temperature in this equation must be measured on the absolute, or Kelvin temperature scale. Using this equation it is possible to measure entropy changes using a calorimeter.

Where S represents entropy, DS represents the change in entropy, q represents heat transfer, and T is the temperature. One useful way of measuring entropy is by the following equation:


 0 kommentar(er)
0 kommentar(er)
